DIET OF Crotalus lepidus (SERPENTES: VIPERIDAE) IN MESA MONTORO, AGUASCALIENTES, MEXICO.
DIETA DE Crotalus lepidus (SERPENTES: VIPERDAE) EN MESA MONTORO, AGUASCALIENTES, MÉXICO.
Rubén Alonso Carbajal-Márquez1* and Gustavo E. Quintero-Díaz1
1Universidad Autónoma de Aguascalientes, Centro de Ciencias Básicas, Departamento de Biología, Avenida Universidad No. 940, Aguascalientes, Aguascalientes 20131, México. *Correspondent: redman031@hotmail.com*
Abstract. This study describes the diet of Crotalus lepidus based on the analysis of the stomach contents and scats of 13 specimens from Mesa Montoro, Aguascalientes, México. We found 14 items belonging to four prey categories. The most important prey category were lizards, with a frequency of occurrence of 61.53% and percentage of appearance of 64.28%, followed by mammals with 23.07% and 21.42% respectively. Also found the category of snake and birds but in a lesser proportion. According to this diverse diet, the data suggests that C. lepidus in Mesa Montoro is a generalist predator in this area.
Keywords. Banded Rock Rattlesnake, Sierra Fría, preys.
Resumen. En este estudio se describe la dieta de Crotalus lepidus con base al análisis de contenidos estomacales y excretas provenientes de 13 ejemplares de Mesa Montoro, Aguascalientes, México. Se encontraron 14 elementos pertenecientes a cuatro categorías de presa. La categoría más importante fueron las lagartijas, con una frecuencia de ocurrencia de 61.53% y un porcentaje de aparición de 64.28%, seguido por los mamíferos con un 23.07% y 21.42% respectivamente. También se encontró la categoría de serpientes y aves pero en menor proporción. De acuerdo a esta diversa dieta, los datos sugieren que C. lepidus en Mesa Montoro es un depredador generalista.
Palabras clave: Cascabel Bandeada de las Rocas, Sierra Fría, presas.
Data of species diet is useful for understanding reproductive strategies, habitat use, distribution patterns, evolutionary divergence in the feeding ecology, as well as providing data for proposing conservation strategies (Taylor, 2001). The encounter with snakes in the wild is irregular and even more difficult to observe an animal in the process of feeding. Hence, there is limited information on diet and predation behavior based on field observations (Bernarde et al., 2000). The composition of the diet is closely related to habitat, and thus their microhabitat preference is related the location of their prey (Hartmann, 2005).
This is why the diet studies are particularly important for understanding the biology of snakes, since it is the primary force in the evolution of morphology and behavior (Holycross et al., 2002). The usefulness of studies of diet composition depends on the accuracy of identification of ingested prey. Because many snakes consume large prey, rarely, most of the snakes captured have empty stomachs. Individuals with stomach contents also typically contain one or a few items, thus reducing the power of statistical comparisons (Halstead et al., 2008). Rattlesnakes generally feed on vertebrates, although they eat invertebrates when they are young (Campbell and Lamar, 2004). Because of this change, the diet of specialized predators may vary with age and size, between sexes and between individuals, depending on seasonal and geographical location (Greene, 1997). In Aguascalientes, Mexico, there is limited detailed information on the biology of most of the snakes, hence natural history studies are needed to generate information useful for conservation of species. Here we describe the composition of the diet of Crotalus lepidus in Mesa Montoro, Aguascalientes, Mexico, with the aim of increasing knowledge about the natural history of the snakes in the state.
METHODS
Fieldwork was conducted in the locality of Mesa Montoro (21°.995728°N, -102.576407°W; datum WGS84) 2406 m elev., located south of the Sierra Fria, between the Municipality of Calvillo and Jesús María, Aguascalientes, México. The dominant vegetation is oak forest (Quercus sp.,) with patches of shrubs (Arctostaphylus pungens, Arbutus glandulosa) and grassland (Bouteloua sp., Mulenberghia sp.) with anthropogenic modifications such as livestock and agriculture. Twenty-three surveys were conducted between January 2008 and March 2009, with a team of three people accumulating 700 hours of effort. The search was conducted in the microhabitat available. When a snake was found, the snout-vent length was measured as well as tail length, weight, sex, number of segments and shape of the rattle. The snake was palpated in search of stomach contents or scats obtained by forced regurgitation and gentle palpation of the last third of body, respectively (Fitch, 1987). The date of capture of each snake was recorded and air temperature, relative humidity was measured and georeferencing with help of a GPS (Garmin). All the snakes were released. The contents and scats obtained were identified using keys and the reference collection of the Vertebrates Collection of UAA (Moore et al., 1974; Knox and Manning, 1992; Monroy-Vilchis and Rubio-Rodríguez, 2003). To determine the diet, the frequency of occurrence was calculated, expressed as the percentage of samples where a prey species appears relative to the number of total samples. To consider the importance of all species, the percentage of appearance was calculated, expressed as the percentage of samples where a prey species appears relative to the number of total appearances (Aranda et al., 1995).
RESULTS
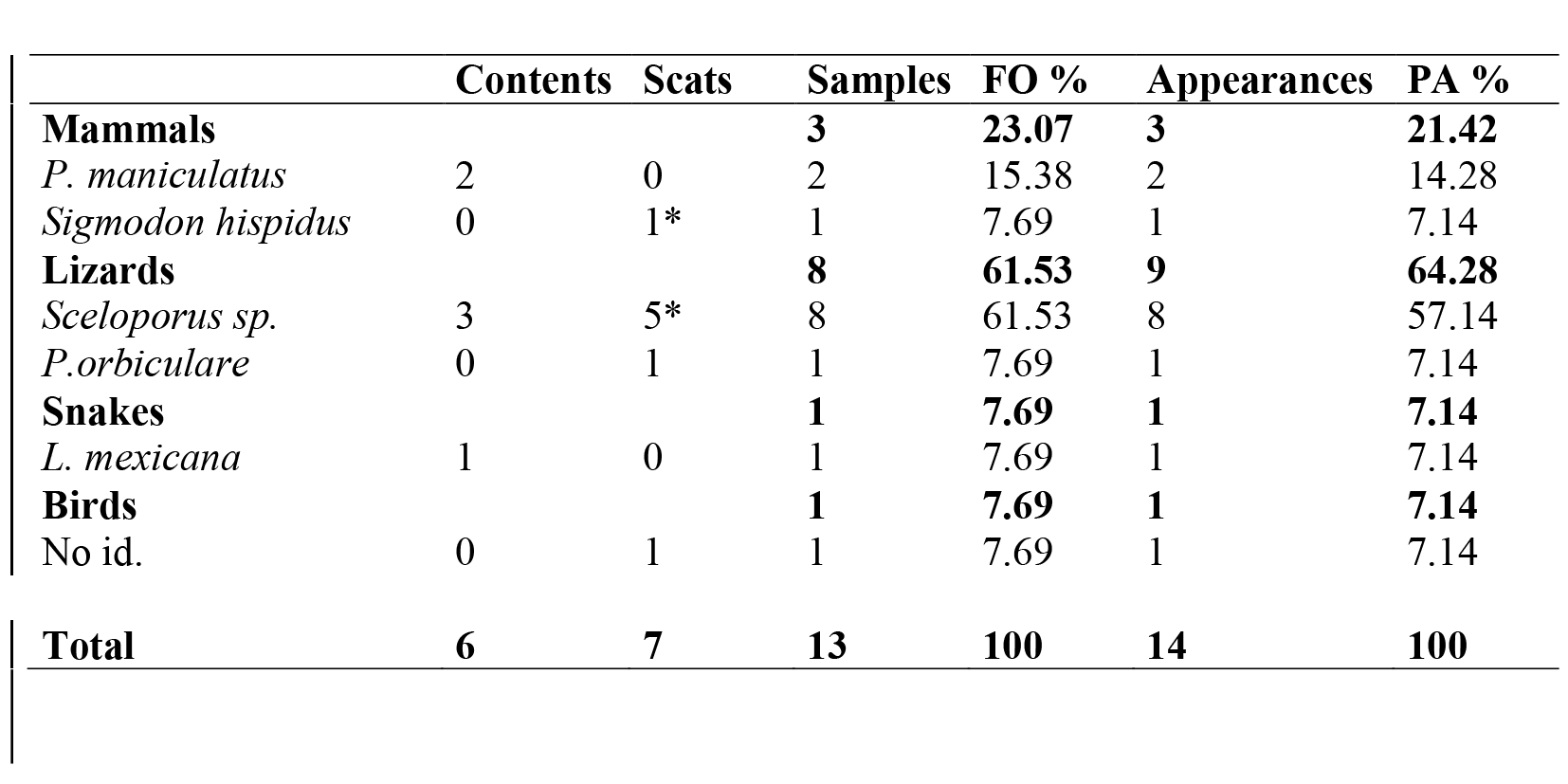
A total of 34 snakes were examined, of which 21 (61.7%) were found not to have contents or scats, six (17.6%) had stomach contents (one male, three females and two juveniles). Six items were found, two (33.3%) Peromyscus maniculatus (males), one (16.6%) lizard Sceloporus torquatus (female), two (33.3%) Sceloporus grammicus (males) and one (16.6%) snake Lampropeltis mexicana (female). All (100%) of the preys were ingested head first. Similarly, the snakes were palpated in search of scats. Of these, seven (20.5%) contained samples (four males and three females) all identifiable items. Eight items were found in seven samples, and only one snake had two types of items. Two (25%) Sceloporus jarrovii, one (12.5%) S. scalaris, one (12.5%) S. grammicus, one (12.5%) S. torquatus, one (12.5%) Phrynosoma orbiculare, one (12.5%) gopher Sigmodon hispidus and one (12.5%) bird, probably a passerine. The values of frequency of occurrence and percentage of appearance are shown in Table I.
DISCUSSION
The results show that C. lepidus has a marked preference for eating lizards, in particular of the genus Sceloporus. This preference is consistent with that indicated by Werler and Dixon (2002) and Ernst and Ernst (2003) on diet of C. lepidus. Sceloporus sp. accounted for 57.14% of the diet of C. lepidus in Mesa Montoro, this coincides with the results obtained by Holycross et al. (2002), stating that only S. jarrovii comprises 45% of total prey. This preference for Sceloporus sp. also was reported by several other authors (Campbell and Lamar, 2004; Vázquez-Díaz and Quintero-Díaz, 2005; Lemos-Espinal and Smith, 2008; Lemos-Espinal and Smith, 2009). In the case of Phrynosoma orbiculare, Ernst and Ernst (2003) and Campbell and Lamar (2004) mentioned that C. lepidus eat lizards of this genus occasionally, but this is the first record of this species (Carbajal-Márquez et al., 2012). Since C. lepidus inhabits rocky and rough places (Werler and Dixon, 2000; Holycross et al., 2002; Ernst and Ernst, 2003; Campbell and Lamar, 2004; Vázquez-Díaz y Quintero-Díaz, 2005) which agrees with our observations, may explain the higher percentage of occurrence of the genus Sceloporus in the diet, as these lizards are found in rocky sites. A large number of C. lepidus were found in stone walls built to divide land property, probably due to the fact that large number of Sceloporus lizards are found concentrated there.
It has been reported that C. lepidus consume mammals to a lesser extent (Werler and Dixon, 2000; Holycross et al., 2002; Ernst and Ernst, 2003; Campbell and Lamar, 2004; Vázquez-Díaz and Quintero-Díaz, 2005), this is reflected in the results, where mammals represent 24.9% of the diet. Holycross et al. (2002) mentions Peromyscus boylii as the most frequently consumed mammal by C. lepidus in many of the habitat it occupies, P. boylii was found in the study area, but does not appear as prey of C. lepidus , in its place P. maniculatus was found. A higher predation of lizards over the rodents can be explained probably by the site location of the study, it is above 2300 m elev., which probably causes the snakes to be mainly active during the day, and to a lesser extent during the night (reaching minimum temperatures of 8°C in the warmer months), so that its activity overlaps more with lizards to a lesser extent with rodents.
Neonates, juveniles and some adults have yellow at the tip of the tail, which can function as a lure to attract lizards, this pigment shows an adaptation to deceive and with this strategy consume this type of prey (Werler and Dixon, 2000; Holycross et al. 2002; Ernst and Ernst, 2003; Lemos-Espinal and Smith 2008). Neonates and juveniles were found mainly in stumps and fallen trees, habitat of Sceloporus grammicus, which was the type of prey found in the neonates. The intake of a snake Lampropeltis mexicana represents 7.14%, suggesting that their intake was occasional, and represent a new item. There are several reports of snake predation and cannibalism by C. lepidus, such as the ingestion of C. lepidus, Gyalopion canum, Virginia striatula and Hypsiglena jani (Werler and Dixon, 2000; Holycross et al., 2002; Ernst and Ernst, 2003; Mata-Silva et al., 2010). The predation on this snake suggests that C. lepidus can use other resources when its main prey is not available. Apparently C. lepidus was able to capture the L. mexicana because they become an easy target especially after recently feeding on a large lizard (Sceloporus torquatus) (Carbajal-Márquez et al., 2012). It has been reported that snakes after feeding display a reduction in activity and mobility and hence are more vulnerable to predation.
The percentage of birds ingested represented the 7.14% and is consistent with some reports (Holycross et al., 2002; Campbell and Lamar, 2004). We found no arthropods, which differs with reports of several other authors, where they mentions the arthropods are prey of juveniles, primarily the centipedes of genus Scolopendra sp. C. lepidus can be considered generalist in relation to different types of prey found in the diet. A high intake of lizards in the study area may be due to its location and activity patterns, high availability of lizards as they share similar microhabitats. Just as habitat modification by the construction of stone walls, which provides refuge to C. lepidus as the lizards.
ACKNOWLEDGMENTS
We thank the Vertebrate Collection, Universidad Autónoma de Aguascalientes. We thank Zaira Yaneth González Saucedo, Edith Alejandra Orozco Medina and J. Jesús Sigala Rodríguez for field help during this study. We also thank SEMARNAT for approving this research (permit number SGPA/DGVS/04324).
Literature cited.
Aranda, M., N. López-Rivera and L. López-de Buen. 1995. Hábitos alimentarios del coyote (Canis latrans) en la Sierra del Ajusco, México. Acta Zoológica Mexicana (n. s.). 65: 89-99.
Bernarde, P.S., J.C., Moura-Leite, R. A., Machado and M.N.C., Kokobum. 2000. Diet of the colubrid snake, Thamnodynastes strigatus (Günther, 1858) from Paraná state, Brazil, with field notes on anuran predation. Revista Brasileira de Biología 60:695-699.
Campbell, J.A. and W. W.Lamar. 2004. The Venomous Reptiles of the Western Hemisphere. Cornell University Press. Volume II. 1032 p.
Carbajal-Márquez R. A., G. E. Quintero-Díaz, Z. Y. González-Saucedo, J. J. Sigala-Rodríguez. 2012. Crotalus lepidus (Banded Rock Rattlesnake). DIET. Herpetological Review 43: 658.
Ernst, C.H. and E. E. Ernst. 2003. Snakes of the United States and Canada. Smithsonian Institution. 668 p.
Fitch, H.S. 1987. Collecting and Life History Techniques in: Seigel, R. A., Collin, J.T., Novac, S.S. (1987): Snakes: Ecology and Evolutionary Biology. Macmillian Publishing Co. 143-164.
Green, H.W.1997. Snakes: The Evolution of Mystery in Nature. University of Carolina Press. 351p.
Halstead, B.J., H.R. Mushinsky and E. D. McCoy. 2008. Sympatric Masticophis flagellum and Coluber constrictor select vertebrate prey at different levels of taxonomy. Copeia (4): 897-908.
Hartmann, P.A. and O.A. Marquez, O.A. 2005. Diet and habitat use of two sympatric species of Phylodryas (Colubridae), in South Brazil. Amphibia-Reptilia. 26: 25-31.
Holycross, A.T., C. W. Painter, D. B. Prival, D. E. Swann, M. J. Schroff, T. Edwards, and C. Schwalbe. 2002. Diet of Crotalus lepidus klauberi (Banded Rock Rattlesnake). Journal of Herpetology. 36: 589-597.
Knox, J.J. and R. W. Manning. 1992 Illustrated Key to Skulls of Genera of North American Land Mammals. Texas Tech University Press. 75 p.
Lemos-Espinal, J.A. and H. M. Smith. 2008. Anfibios y Reptiles del estado de Coahuila, México. Universidad Nacional Autónoma de México. University of Colorado at Boulder. CONABIO. México. 550 p.
Lemos-Espinal, J.A. and H. M. Smith. 2009. Anfibios y Reptiles del estado de Chihuahua, México. Universidad Nacional Autónoma de México. University of Colorado at Boulder. CONABIO. México. 613 p.
Mata-Silva, V., S. Dilks and J.D. Johnson. 2010. Crotalus lepidus (Rock Rattlesnake) DIET. Herpetological Review 41: 235-236.
Moore, T.D., L. E. Spence and C. E. Dugnolle. 1974. Identification of the dorsal guards hairs of some mammals of Wyoming. Wyoming Game and Fish Deparment. U.S.A. 177 p.
Monroy-Vilchis, O., and R. Rubio-Rodríguez. 2003. Guía de Identificación de mamíferos terrestres del Estado de México, a través del pelo de guardia. Universidad Autónoma del Estado de México. México. 115p.
Taylor, E. N. 2001. Diet of the Baja California Rattlesnake, Crotalus enyo (Viperidae). Copeia (2):553-555.
Vázquez-Díaz, J. and G. E. Quintero-Díaz. 2005. Anfibios y Reptiles de Aguascalientes. CONABIO.CIEMA A.C. México. 318p.
Werler, J.E. and J. R. Dixon. 2000. Texas Snakes. University of Texas Press. 437p.